| a = | 1 always | |
| b = | 2 if n≤nir b = 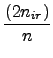 if n > nir if n > nir |
with parallel spin coupling of shells.
This covers e.g. the p5 s1 3P states, or the d4s1
6D states of atoms. The coupling information is given following the
keyword $rohf. The (a, b) within a shell are taken from above
(6.3.2), the cross term (shell 1)-(shell 2) is in
this case:
| a = | 1 always | |
| b = | 2 if n≤nir b = 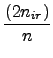 if n > nir if n > nir |